The science behind fireworks: What goes into amazing Victoria Day displays?
Green peonies, blue dahlias, red crossettes, white and silver chrysanthemums; how are all of these incredible fireworks produced?
Victoria Day is fast approaching and numerous celebrations across Canada will include fireworks lighting up the night sky. Here's the science behind these delightful pyrotechnic displays.
Fireworks explode in a variety of sizes, shapes, and colours. Despite this, each is based on combining just two basic components — the sparkler and the firecracker.

A sparkler. (Getty Images)
For many people, their first experience with a sparkler involved impatiently waiting for the fireworks show to begin and being handed a bright, sparking metal stick they could wave around to pass the time. Although we likely didn't know it then, we were holding one of the essential components of the colourful displays we were waiting for.
A sparkler is composed of a dried mixture of chemical compounds stuck to a metal wire handle. The mixture will always contain three specific components: tiny bits of metal (usually aluminum), a chemical oxidizer, such as potassium perchlorate, and some kind of starchy substance, like dextrin, to bind everything together and glue it to the handle. Charcoal or sulphur are sometimes added to provide extra fuel for the sparkler to burn.
The metal bits are the literal stars of the show when a sparkler is lit. They are what produce the sparks as they burn in the presence of oxygen.
However, it's the oxidizer that starts the show and keeps it going to the end.
According to Dr. Joe Schwarcz, the director of McGill University's Office for Science and Society: "In order to produce an intense glow, more oxygen is needed than is available from the surrounding air. This is where the potassium perchlorate comes in. It is an oxidizing agent, meaning that it is a source of oxygen. Potassium chlorate undergoes a chemical reaction, initiated by the lighting of the sparkler, whereby it decomposes to yield potassium chloride and oxygen. The oxygen then combines with the metal, allowing it to burn. This combustion process produces heat, which then decomposes more potassium perchlorate, yielding more oxygen, which then combines with more metal, and on and on until one of the reagents runs out."

A bundle of unlit firecrackers. (Ritesh Man Tamrakar/Flickr. CC BY 2.0)
A firecracker is a sealed paper tube or sphere packed with gunpowder — a mixture of potassium nitrate, charcoal, and sulphur — along with a fuse to deliver a flame to the interior. Rather than just a simple piece of string or cord, firework fuses are quite complex, consisting of different layers of cord wrapped around a gunpowder core, which burn with a visible sparking flame.
When the firecracker is lit, the flame burns down the fuse until it reaches the interior of the paper tube or sphere. There, a chain reaction occurs. The sulphur in the gunpowder catches first, providing a flame to burn the charcoal, which heats the potassium nitrate to release oxygen, which keeps the process going until all of the gunpowder is burned. The heat of all this combustion causes the gases released to expand, which push against the inner wall of the firecracker until it bursts.
The resulting explosion produces a brief flash of flame and releases a pressure wave through the air that registers to our ears and brain as a loud bang. The bigger the firecracker, the bigger the explosion and intensity of the bang.
The show really begins when the concepts of these two types of firework are combined together, though. This results in the firework shell.

Some firework shells can be MUCH more complex, like this roughly metre-wide Japanese shell, which features not only sparkler spheres lining the interior but also nested shells that contain even more sparkler spheres, designed to go off well after the initial burst. (Joel(frikitiki)/Flickr. CC BY-ND 2.0)
The most basic firework shell starts with a hollow cylinder or sphere made of paper and string. A firecracker called the burst charge is placed at the core of the shell and linked to the outside by a timing fuse. Gunpowder is then packed into the remaining space around the burst charge. Embedded within that gunpowder is a collection of small stars, each containing the chemical components of a sparkler.
After the timing fuse is lit, the shell fires into the air from a special launch tube, sometimes using a launch charge incorporated directly into the bottom of the shell. As the shell climbs higher, the ember crawls up the slow-burning timing fuse until it reaches the end, which sets off the burst charge.
The explosion of the burst charge ignites the gunpowder in the shell, which simultaneously blows the shell apart and sets the sparkler stars burning, which are then flung outwards by the force of the detonation.
The colour and shape of the resulting pyrotechnic display depend on the exact makeup of the stars and their precise arrangement within the shell.

A multi-colour firework burst. (DeltaWorks/Pixabay)
Firework colours are produced by using specific metals or metal compounds in the stars. Some of the most common are:
White: aluminum or magnesium
Silver: aluminum, magnesium, or titanium powder
Blue: copper chloride or copper compounds
Red: strontium salts or lithium salts
Green: barium chloride
Yellow: sodium nitrate
Orange: calcium chloride
Purple: a mix of strontium (red) and copper (blue) compounds
These colours result from the atoms of these different elements absorbing excess energy (from burning) and then dumping that excess energy as photons of light. The exact amount of energy carried away by the photon determines the frequency and wavelength of the light, and thus its colour.
In the case of aluminum, photons are emitted across the entire spectrum of colours, which combine to appear white. Other elements are more focused. Copper, for example, releases most of its photons in the energetic blue part of the spectrum, while barium emits mostly green light, and strontium releases mostly red light. Combining different elements together in the same star will result in a combination of their light. Thus, we can produce purple from burning copper and strontium together.

The different metals or chemical compounds that produce the different firework colours. (Base firework image by Artur Strecker/Pixabay, infographic by Scott Sutherland)
When the sparkler stars are packed into a shell randomly, it results in a spherical display known as a peony, named after the flower.
Firework designers can cleverly shape displays to their wishes, though. By combining different coloured stars, different sparkler mixtures, and carefully placing the stars, they can produce very specific patterns. This is how we get other flower-like displays, such as the dahlia, chrysanthemum, and pistil.

This composite image shows (left to right) peony, chrysanthemum, dahlia, and pistil displays. (Images by Tuan Hung Nguyen/Pixabay, composite by Scott Sutherland)
Tree-like palm and willow displays are also popular, and patterns can be arranged to produce rings, stars, spiders, waterfalls, and a host of other shapes as well.
Special effects can be achieved by organizing layers of gunpowder and stars that go off at different times.
One way of accomplishing this is by packing a larger shell with several smaller shells. Another way is to change the composition of the stars so that they burn longer, crackle and whistle as they burn, or burn down and then expose a secondary shell that explodes.

This New Year's fireworks display shows off the variety of possible patterns and colours. (Nick/Pixabay)
And the really great thing is that knowing the science that goes into making these fantastic displays takes absolutely nothing away from the awe they inspire!
Weather and fireworks
Just like with any other event occurring in the sky — celestial or terrestrial — weather can have a big impact on the enjoyment of a fireworks show. The weather forecast can even prompt organizers to postpone or cancel a show.
Clouds typically are not much of a hindrance to a fireworks display. The smallest firework shells explode around 120 metres up, while the largest detonate around 350 metres above the ground. Thus, unless there is thick fog or a very low layer of stratus clouds, the fireworks show will remain in full view of the crowd.

(kazuend/Unsplash)
Wind is the most common concern for fireworks — both the wind direction and how hard the wind is blowing.
Partly, this is a matter of ensuring the wind does not cause firework shells to stray beyond where they are supposed to go off. However, wind can also carry the smoke and other debris from the firework shells across the assembled crowd, which can be hazardous to their health.
The same holds true for a lack of wind, as the stagnant air becomes thicker with smoke as the show progresses. That would make it more difficult to see the firework displays, but would also pose an air quality health risk to those in attendance.
This lack of wind can also be a problem the morning after a fireworks show. This is due to a weather phenomenon known as a temperature inversion.
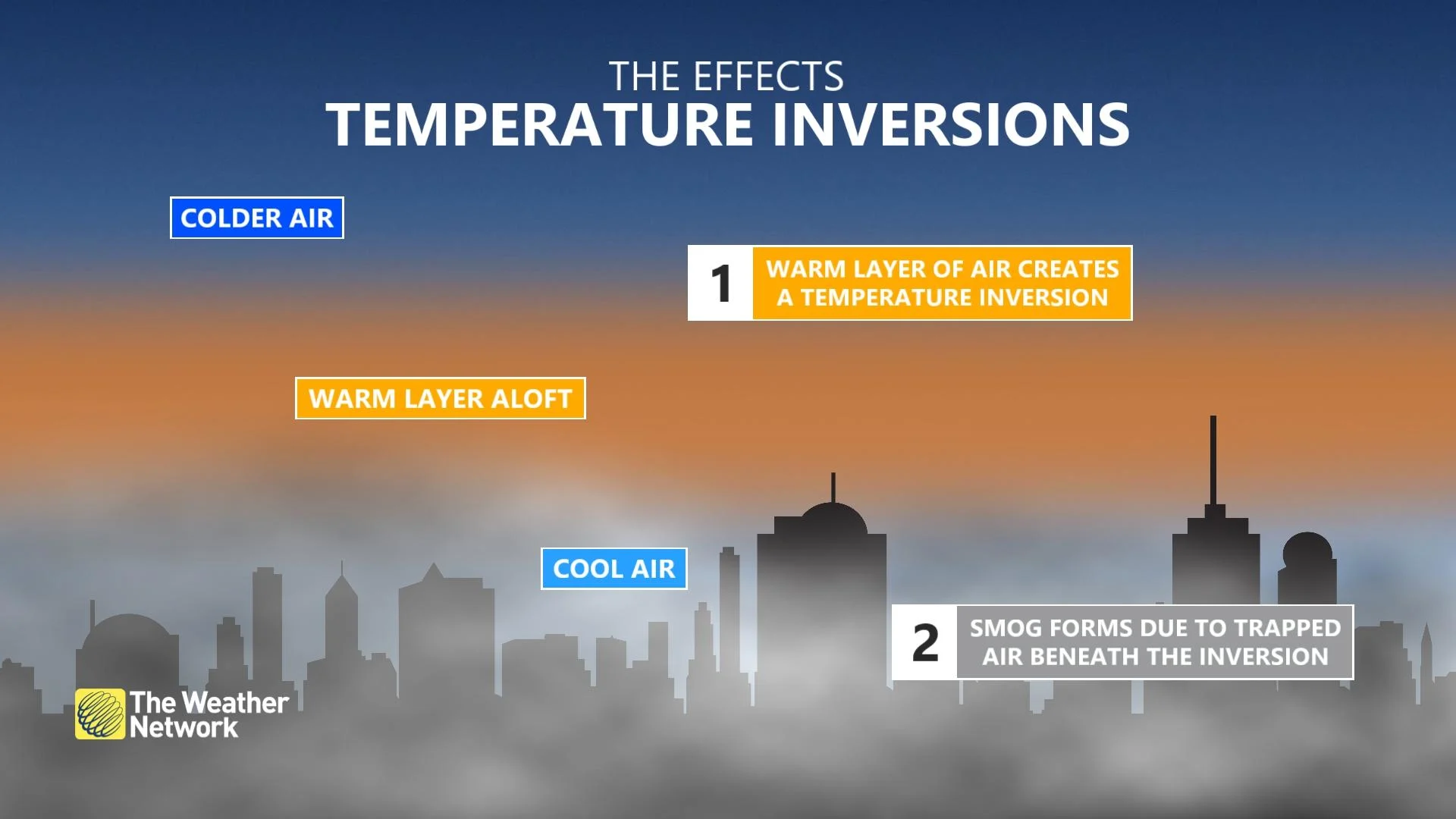
An inversion can be a concern on any day of the year. It effectively cuts off the air closest to the ground from the rest of the atmosphere. Thus, it limits the volume of air available for pollution from cars, industry, and power generation to mix into. This results in these pollutants becoming more concentrated, the longer the inversion persists.
However, the day after a fireworks show can be even worse, as the smoke produced during the event lingers and mixes in with the typical urban air pollution to degrade the air quality even further.
Weather Forecast
In case the weather in your area may impact the local firework show, here are the precipitation and low-cloud forecasts for Sunday and Monday evening.
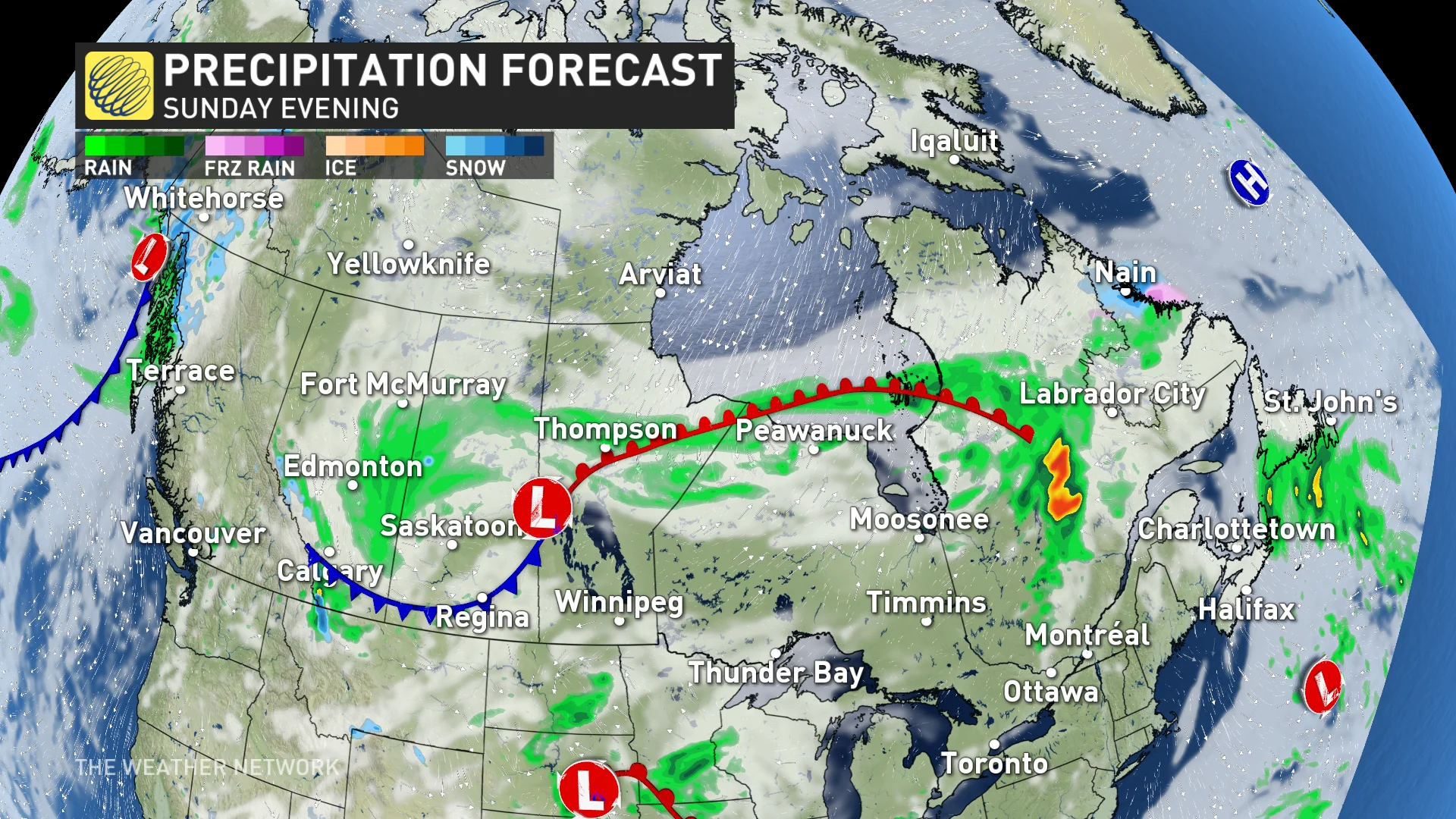
(Canada's forecast for Sunday, May 19)
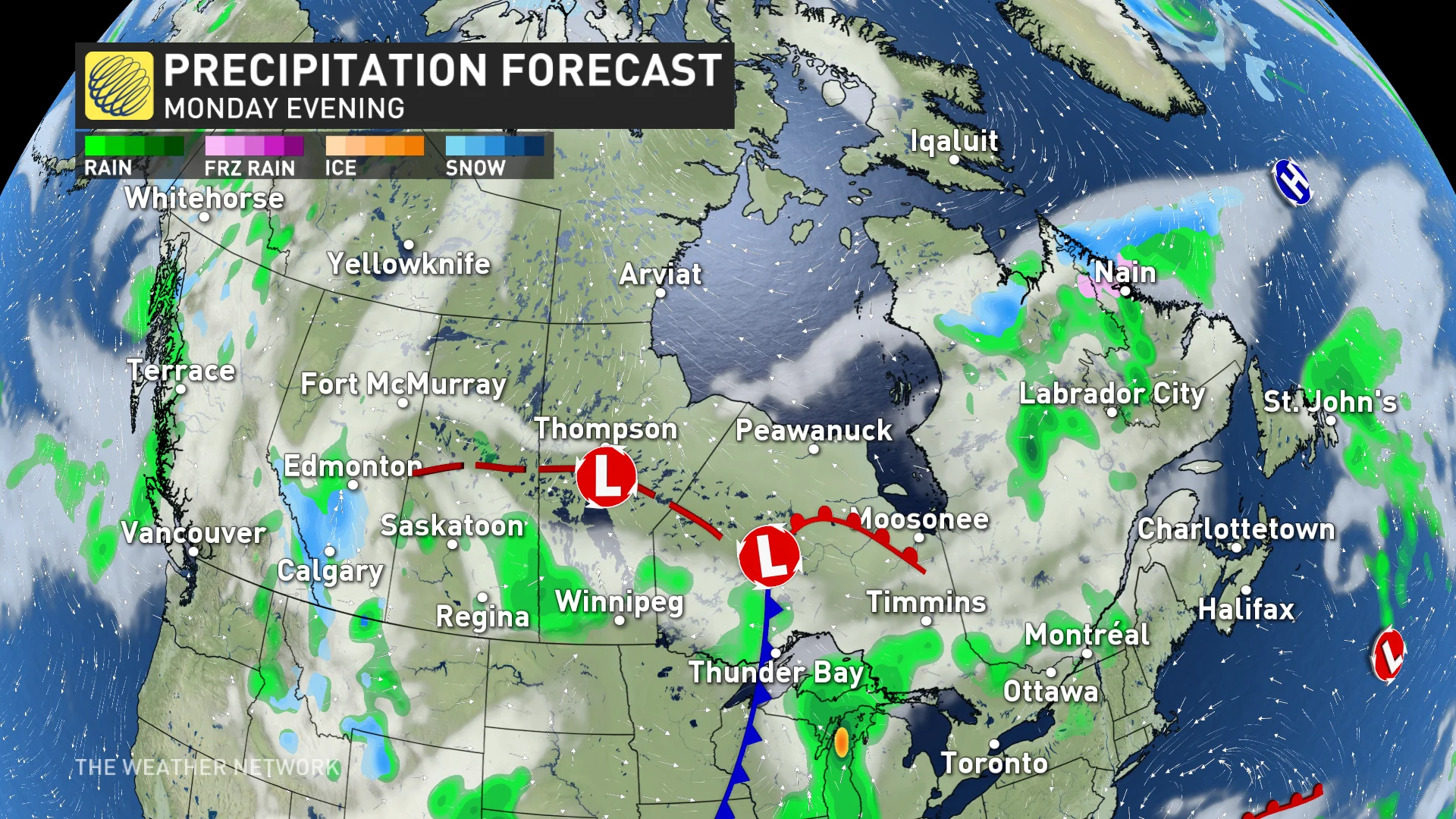
(Canada's forecast for Monday, May 20, Victoria Day)
Fire bans and firework bans
Many municipalities have firework bans in their bylaws, restricting both the local sale of fireworks and the personal use of fireworks. Check your local community's rules, just to be sure.
Besides personal safety and noise complaints, though, the use of fireworks is often included in fire bans that are issued due to drought conditions.
Right now, much of the western half of Canada is experiencing some level of drought.
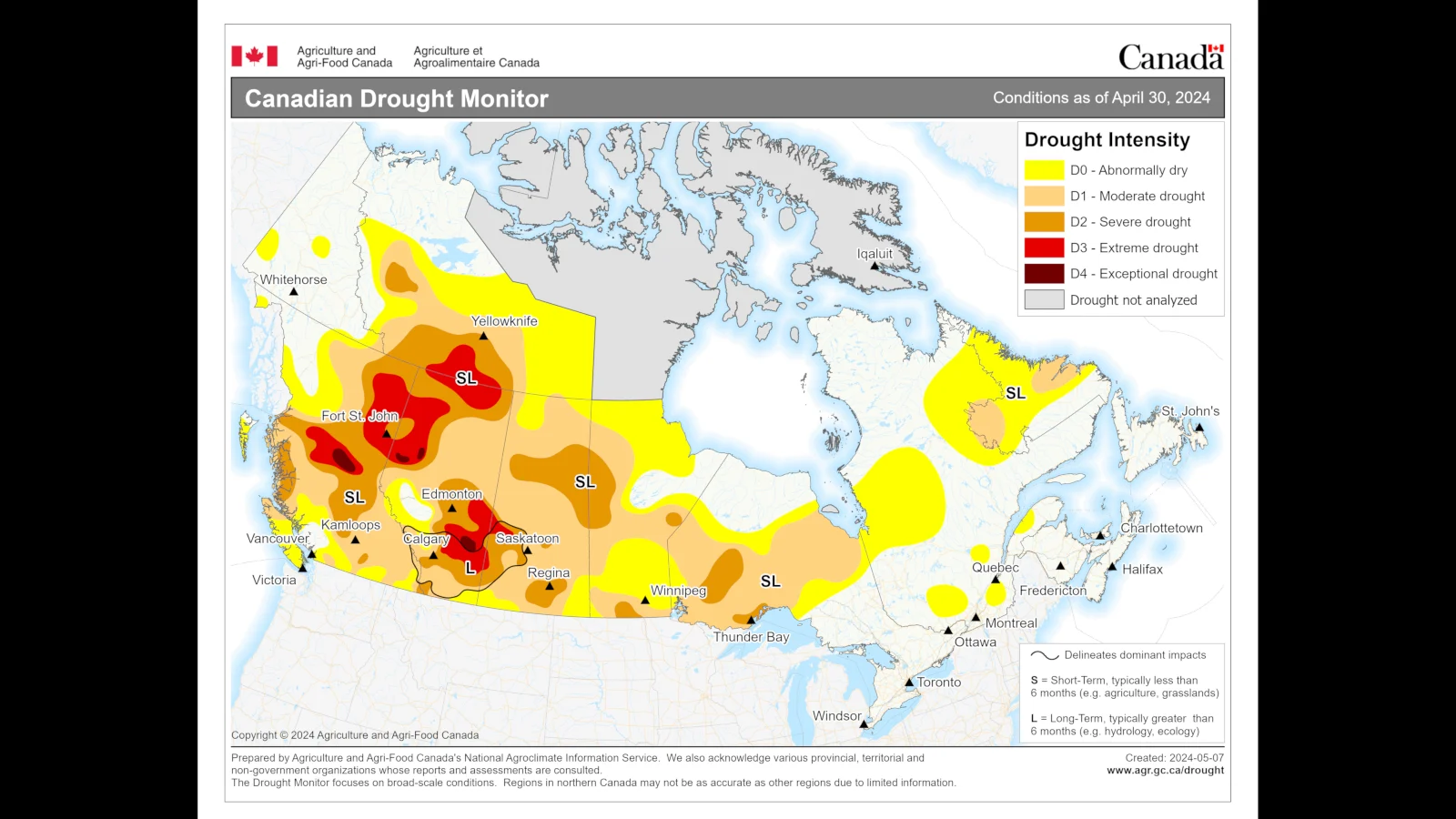
Drought conditions across Canada as of April 30, 2024. (Agriculture and Agri-Food Canada)
Because of these dry conditions, four of British Columbia's six Fire Centre regions — Cariboo, Coastal, Kamloops, and Prince George — have a complete ban on any kind of open fire larger than a campfire (this includes the use of fireworks). Bans have also been issued in the southwestern areas of the province's Northwest Fire Centre.
In Alberta, there are numerous fire advisories and fire restrictions throughout the province, each of which prohibits firework use without the written permission of a Forest Officer. Current fire bans, as of May 15, are located in specific communities around the city of Edmonton and in the Grand Prairie Forest Area. Alberta Provincial Parks also has a number of fire bans in place on the lands they manage.
Saskatchewan has a scattering of fire bans in place, all of which are in regions located north of Saskatoon — Barrier Valley, Arborfield, North Battleford, Garden River, Leoville, Eldin, and Frenchman Butte. Fire restrictions have also been issued in Wilton, Loon Lake, and Meadow Lake Provincial Park.
In Manitoba, there are burning restrictions in place in a few municipalities throughout the province. However, only the fire ban in the town of Grand Rapids specifically includes no fireworks.